Natural products have become a rich source of anti-cancer lead compounds due to the diversity of their structure and mechanism of action. In May 2011, Korean and Japanese scientists Ahn, Osada, and Kim collaborated on the separation, structural identification, and biological activity studies of the natural product fusarisetin A (J. Am. Chem. Soc. 2011, 133, 6865). Unlike the more common small anti-cancer molecules (such as tubulin stabilizers / destabilizers and DNA topoisomerase inhibitors), this molecule shows a strong effect of inhibiting the migration and penetration of cancer cells, while there is no obvious Cytotoxicity. From the structural point of view, the molecule has 10 chiral centers on the 6,6,5,5,5 paracyclic skeleton, which has certain synthesis challenges.
Total synthesis of Fusarisetin A
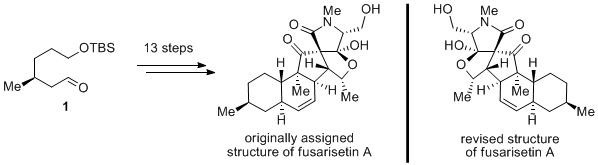
Researchers Lu Zhaoyong, Deng Jun, Zhu Bo, Yu Haixin of the State Key Laboratory of Bioorganic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, led by Researcher Li Ang, completed the original structure of fusarisetin A after 6 months of hard work The first total synthesis (J. Am. Chem. Soc. 2012, 134, 920). Although the spectral properties of the synthetic compound and the natural product are consistent with the specific optical rotation values, the optical rotation directions of the two are just opposite, so the absolute stereo configuration of the natural product is corrected, that is, the true structure should be the original structure Of the enantiomers.
From a chemical point of view, the synthetic route starts from the known aldehyde 1 and undergoes 13 steps of conversion to the target molecule (–)-fusarisetin A, as shown in the figure. The key steps include the intramolecular Diels-Alder reaction promoted by Lewis acid, palladium-catalyzed allyl oxygen-carbon replacement, position-selective Wacker oxidation, and finally the cascade process of Dieckmann condensation / hemipalization.
This work not only determines the absolute stereoconfiguration of natural fusarisetin A, but also prepares for the synthesis of analogues of this natural product and the study of its target and mechanism of action. At the same time, some synthetic strategies are expected to be applied to other structurally related natural Product synthesis research.
This work was greatly supported by the Chinese Academy of Sciences, the National Natural Science Foundation of China, the Shanghai Municipal Science and Technology Commission, the Shanghai Institute of Organic Chemistry, and the National Key Laboratory of Life Organics.
2 Tier Iron Chrome Plated Dish Rack
2 Tier Iron Chrome Plated Dish Rack,2Tier Dish Rack,2Tier Chrome Plating Iron Dish Rack,Chrome Plating Iron Dish Drying Rack
Jiangmen Jianghai Jianshang Houseware Co.,LTD. , https://www.jianshanghouseware.com